| ISF Master Reference List_DO NOT EDIT_downloaded.xlsm |
|---|
|
|---|
| A | B | C | D | E | F | G | H | I | J | K |
|---|
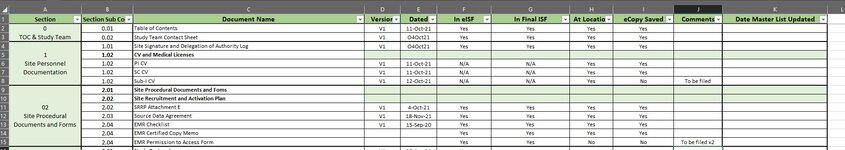
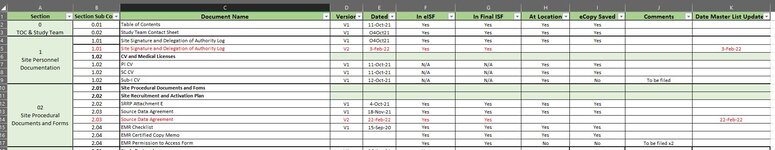
| 1 | Section | Section Sub Code | Document Name | Version | Dated | In eISF | In Final ISF | At Location | eCopy Saved | Comments | Date Master List Updated |
|---|
| 2 | 0
TOC & Study Team Contact Sheet | 0.01 | Table of Contents | V1 | 11-Oct-21 | Yes | Yes | | | | |
|---|
| 3 | 0.02 | Study Team Contact Sheet | V1 | O4Oct21 | Yes | Yes | | | | |
|---|
| 4 | 1
Site Personnel Documentation | 1.01 | Site Signature and Delegation of Authority Log | V1 | O4Oct21 | Yes | Yes | | | | |
|---|
| 5 | 1.02 | CV and Medical Licenses | | | | | | | | |
|---|
| 6 | 1.02 | PI CV | | | | | | | | |
|---|
| 7 | 1.02 | SC CV | | | | | | | | |
|---|
| 8 | 1.02 | Sub-I CV | | | | | | | | |
|---|
| 9 | 02
Site Procedural Documents and Forms | 2.01 | Site Procedural Documents and Foms | | | | | | | | |
|---|
| 10 | 2.02 | Site Recruitment and Activation Plan | | | | | | | | |
|---|
| 11 | 2.02 | SRRP Attachment E | V1 | 4-Oct-21 | Yes | Yes | | | | |
|---|
| 12 | 2.03 | Source Data Agreement | V1 | 18-Nov-21 | Yes | Yes | | | | |
|---|
| 13 | 2.04 | EMR Checklist | V1 | 15-Sep-20 | Yes | Yes | | | | |
|---|
| 14 | 2.04 | EMR Certified Copy Memo | | | Yes | Yes | | | | |
|---|
| 15 | 2.04 | EMR Permission to Access Form | | | Yes | Yes | | | | |
|---|
| 16 | 03
Study Protocol | 3.01 | Study Protocol | V1 | 26-Apr-21 | Yes | | | | | |
|---|
| 17 | 3.01 | Final and Amendments | | | | | | | | |
|---|
| 18 | 3.01 | Protocol Singature Page | V1 | 18-Aug-21 | Yes | | | | | |
|---|
| 19 | 3.01 | Study Schedule Flowchart | | | | | | | | |
|---|
| 20 | 3.01 | Inclusion/Exclusion Checklist | V1 | 18-Oct-21 | Yes | | | | | |
|---|
| 21 | 3.02 | Study Procedure Manuals | | | | | | | | |
|---|
| 22 | 3.02 | 6MWT guideline & checklist | V1 | 9-Sep-21 | Yes | | | | | |
|---|
| 23 | 3.02 | Blank eCRF | V1 | 5-Oct-21 | | | | | | |
|---|
| 24 | 3.02 | EDC Completion Guidelines | V1 | 5-Oct-21 | Yes | | | | | |
|---|
| 25 | 3.02 | RHC Guideline | | | N/A | N/A | | | | |
|---|
| 26 | 3.02 | RHC Worksheet | V1 | 12-Aug-21 | N/A | N/A | | | | |
|---|
| 27 | 04
ICF and Subject Materials | 4.01 | Informed Consent, including translations (if any) | | | | | | | | |
|---|
| 28 | 4.01 | Main ICF V1 | V1 | 15-Sep-21 | | | | | | |
|---|
| 29 | 4.01 | Newborn ICF V1 | V1 | 15-Sep-21 | | | | | | |
|---|
| 30 | 4.01 | Greenphire ICF V1 | V1 | 10-Sep-21 | | | | | | |
|---|
| 31 | 4.01 | Pregnant Partner NTF from Advarra | V1 | 9-Nov-20 | Yes | Yes | | | | |
|---|
| 32 | 4.01 | Subject Information Sheet and Informed Consent Form | | | | | | | | |
|---|
| 33 | 4.01 | Signed Informed Consent Forms, if not filed with Source Documents or under 19.02 | | | | | | | | |
|---|
| 34 | 4.02 | Subject Materials, including translations (if any) | | | | | | | | |
|---|
| 35 | 4.02 | Patient Letter | V1 | 20-Jul-21 | Yes | Yes | | | | |
|---|
| 36 | 4.02 | PCP Participant enrollment letter | V1 | 20-Jul-21 | Yes | Yes | | | | |
|---|
| 37 | 4.02 | Recruitment poster | V1 | 8-Jul-21 | Yes | Yes | | | | |
|---|
| 38 | 4.02 | Study introduction trifold (for subjects) | V1 | 22-Jul-21 | Yes | Yes | | | | |
|---|
| 39 | 4.02 | Thank You Card | V1 | 20-Jul-21 | Yes | Yes | | | | |
|---|
| 40 | 4.02 | Patient Card | V1 | 2-Jul-21 | Yes | Yes | | | | |
|---|
| 41 | 4.02 | Borg CR10 Instructions | V1 | N/A | Yes | Yes | | | Not Printed in Binder - Provided by Jumo Health | |
|---|
| 42 | 4.02 | Borg CR10 Scale | V1 | N/A | Yes | Yes | | | Not Printed in Binder - Provided by Jumo Health | |
|---|
| 43 | 4.03 | Greenphire Travel and Reimbursements | | | | | | | Also available in Spanish if applicable | |
|---|
| 44 | 4.03 | Greenphire ClinCard Cardholder RideShare FAQ | V1 | 18-Apr-19 | Yes | Yes | | | | |
|---|
| 45 | 4.03 | Greenphire ClinCard Carrier English | V5 | Sep-20 | Yes | Yes | | | | |
|---|
| 46 | 4.03 | Greenphire ClinCard Travel Ref Guide | V3 | 11-Oct-16 | Yes | Yes | | | | |
|---|
| 47 | 4.03 | Greenphire ConneX USA Travel Contact Card | V1 | Jan-19 | Yes | Yes | | | | |
|---|
| 48 | 5
Regulatory | 5.01 | Site Specific Regulatory Submission/Approval/Acknowledgement | | | Folders are present but all information within will be provided and filed by site | Folders are present but all information within will be provided and filed by site | | | | |
|---|
| 49 | 5.02 | FDA 1572 | V1 | 11-Aug-21 | | | | |
|---|
| 50 | 5.03 | Financial Disclosure | | | | | | |
|---|
| 51 | 5.03 | PI FDF - | V1 | 11-Aug-21 | | | | |
|---|
| 52 | 5.03 | Sub-I FDF - | V1 | | | | | |
|---|
| 53 | 5.03 | Sub-I - | V1 | | | | | |
|---|
| 54 | 5.04 | Other Site Specific Approvals | | | | | | |
|---|
| 55 | 5.05 | Regulatory Correspondence | | | | | | |
|---|
| 56 | 6
Ethics (IRB) | 6.01 | Ethics Initial CTA Submission/Approval/Acknowledgment | | | Folders are present but all information within will be provided and filed by site | Folders are present but all information within will be provided and filed by site | | | | |
|---|
| 57 | 6.02 | Ethics Amendment CTA Submission/Approval/Acknowledgment | | | | | | |
|---|
| 58 | 6.03 | Ethics Progress/Periodic/Clinical Study Reports Submission/Approval/Acknowledgment | | | | | | |
|---|
| 59 | 6.03 | Initial IRB Approval | | | | | See IRB Letters Tab | |
|---|
| 60 | 6.03 | Ethics Study Deviation(s) Submission/Approval/Acknowledgment | | | | | | |
|---|
| 61 | 6.03 | Ethics SAE Submission/Approval/Acknowledgment | | | | | | |
|---|
| 62 | 6.04 | Ethics Expedited Safety Report Submission/Approval/Acknowledgment | | | | | | |
|---|
| 63 | 6.05 | End of Study Submission/Approval/Acknowledgment | | | | | | |
|---|
| 64 | 6.06 | Ethics GCP Compliance Statement | | | | | | |
|---|
| 65 | 6.07 | Ethics Documenatation of Non-Voting Status (if not inlcuded in Submission/Approval/Acknowledgment) | | | | | | |
|---|
| 66 | 6.08 | Ethics Correspondence | | | | | | |
|---|
| 67 | 7
Legal & Financial Documentation | 7.01 | CDA | | | Folders are present but all information within will be provided and filed by site | Folders are present but all information within will be provided and filed by site | | | | |
|---|
| 68 | 7.02 | Indemnification | | | | | | |
|---|
| 69 | 7.03 | Clinical Trial Agreement(s) Investigator and site, including translations | | | | | | |
|---|
| 70 | 7.04 | Clinical Trial Agreement(s) Site Departments and Service Providers, including translations | | | | | | |
|---|
| 71 | 7.05 | Other Legal and Financial documentation (W9, DPA, PAF if not included in CTA) | | | | | | |
|---|
| 72 | 7.06 | Invoices and Payment Information | | | | | | |
|---|
| 73 | 7.07 | Insurance | | | | | | |
|---|
| 74 | 7.08 | Legal and Financial Corespondence | | | | | | |
|---|
| 75 | 8
Safety | 8.01 | Investigator Brochure/Investigational Medicinal Product Dossier/SPC, translations (if any) | V16 | 17-Apr-21 | Yes | Yes | | | | |
|---|
| 76 | 8.02 | SAE and Pregnancy reporting forms | | | | | | | | |
|---|
| 77 | 8.02 | Exposure During Pregnancy Reporting Form | V1 | N/A | Yes | Yes | | | | |
|---|
| 78 | 8.02 | SAE Reporting Form | V1 | N/A | Yes | Yes | | | | |
|---|
| 79 | 8.03 | Expedited Safety Reports | | | | | | | | |
|---|
| 80 | 8.04 | PsiXchange Reference Guideline | | | Yes | Yes | | | | |
|---|
| 81 | 9
Laboratory (Central and Local) | 9.01 | Laboratory Manual (Central laboratory) | V1 | 13-Nov-21 | Yes | Yes | | | | |
|---|
| 82 | 9.01 | Reference Ranges | | | | | | | | |
|---|
| 83 | 9.01 | CV of Laboratory Head | | | | | | | | |
|---|
| 84 | 9.01 | PPD Laboratories Collection Flow Chart | V1 | 14-Sep-21 | Yes | Yes | | | | |
|---|
| 85 | 9.02 | Reference Rangse/Normal Values (Local Lab) | N/A | 24Sug21 | | | | | | |
|---|
| 86 | 9.03 | Accreditation Records and/or Head of Laboratory CV (Local laboratory) | | | | | | | | |
|---|
| 87 | 9.04 | Training / Meeting Materials | | | | | | | | |
|---|
| 88 | 9.04 | Preclarus Lab Data Portal User Guide | V2 | 20-Jul-18 | Yes | Yes | | | | |
|---|
| 89 | 9.05 | Bio-analytical Documentation | | | | | | | | |
|---|
| 90 | 9.06 | Labeling | | | | | | | | |
|---|
| 91 | 9.07 | Lists/Logs (e.g. record of retained body fluids/tissue samples (if any), | | | | | | | | |
|---|
| 92 | 9.07 | Lab Samples Storage Temp Log | V1 | 11-Oct-21 | Yes | Yes | | | | |
|---|
| 93 | 9.07 | Lab Samples Storage_Subject-Specific | V1 | 11-Oct-21 | Yes | Yes | | | | |
|---|
| 94 | 9.07 | Shipment records | | | | | | | | |
|---|
| 95 | 9.08 | Laboratory Correspondence | | | | | | | | |
|---|
| 96 | 10
Vendors/Local Department(s)/Third Party Provider(s) | 10.01 | Almac IRT | | | | | | | | |
|---|
| 97 | 10.01 | Vendor Manual/ IxRS User Guides | V1 | 5-Nov-21 | Yes | Yes | | | | |
|---|
| 98 | 10.01 | Training/Meeting Materials | | | | | | | | |
|---|
| 99 | 10.01 | Lists/Logs/Vendor Correspondence | | | | | | | | |
|---|
| 100 | 10.02 | BAIM Institute | | | | | | | | |
|---|
| 101 | 10.02 | BAIM Manual | V1 | 3-Nov-21 | Yes | Yes | | | | |
|---|
| 102 | 10.02 | Training/Meeting Materials | | | | | | | | |
|---|
| 103 | 10.02 | Lists/Logs/Vendor Correspondence | | | | | | | | |
|---|
| 104 | 10.03 | BioTel Research | | | | | | | | |
|---|
| 105 | 10.03 | ECG acquisition guide | V1 | 3-Aug-21 | Yes | Yes | | | | |
|---|
| 106 | 10.03 | ECG site information form | V1 | 17-Jun-21 | Yes | Yes | | | | |
|---|
| 107 | 10.03 | ECHO data transmittal form | V1 | 30-Aug-21 | Yes | Yes | | | | |
|---|
| 108 | 10.03 | ECHO participant screening guide | V1 | 30-Aug-21 | Yes | Yes | | | | |
|---|
| 109 | 10.03 | ECHO participant screening guide | V2 | 8-Feb-22 | Yes | Yes | | | | 15-Feb-22 |
|---|
| 110 | 10.03 | ECHO quick reference guide | V1 | 27-Jul-21 | Yes | Yes | | | | |
|---|
| 111 | 10.03 | ECHO site questionnaire | V1 | 23-Aug-21 | Yes | Yes | | | | |
|---|
| 112 | 10.03 | Ambra eTransfer Site User Guide | V1 | N/A | | | | | | |
|---|
| 113 | 10.03 | QMS Site User Guide | V1 | 10-Oct-20 | | | | | | |
|---|
| 114 | 10.03 | Site Validation/Qualification | | | | | | | | |
|---|
| 115 | 10.03 | ECG Test Transmission Successful Email | | No | | Yes | | | | |
|---|
| 116 | 10.03 | ECHO - First Participant Ready Notification | | No | | Yes | | | | |
|---|
| 117 | 10.03 | Lists/Logs/Vendor Correspondence | | | | | | | | |
|---|
| 118 | 10.03 | ECHO Peer Training Log | V3 | 2-Jun-20 | Yes | Yes | | | Note: It says to fax a copy of the log to BTR at the bottom of the form, this is not required. The site maintains the log for their records | 24-Feb-22 |
|---|
| 119 | 10.03 | ECG Peer Training Log | V3 | 2-Jun-20 | Yes | Yes | | | Note: It says to fax a copy of the log to BTR at the bottom of the form, this is not required. The site maintains the log for their records | 24-Feb-22 |
|---|
| 120 | 10.04 | Greenphire | | | | | | | | |
|---|
| 121 | 10.04 | Welcome to Clincard | V1 | N/A | Yes | Yes | | | | |
|---|
| 122 | 10.04 | Welcome to ConneX | V1 | Feb-20 | Yes | Yes | | | | |
|---|
| 123 | 10.04 | ClinCard Reference Guide for Coordinators | V7.3 | Oct-19 | Yes | Yes | | | | |
|---|
| 124 | 10.04 | ConneX Travel Reference Guide for Coordinators | V2 | Dec-21 | Yes | Yes | | | | |
|---|
| 125 | 10.04 | Grenphire New User Training Handout | N/A | N/A | Yes | Yes | | | | |
|---|
| 126 | 10.04 | Greenphire Generic ClinCard | V3 | Jun-18 | Yes | Yes | | | | |
|---|
| 127 | 10.04 | Greenphire ClinCard Messgae Template | V5 | Feb-18 | Yes | Yes | | | | |
|---|
| 128 | 10.04 | Greenphire CliCard Rideshare Message Template | V1 | Apr-19 | Yes | Yes | | | | |
|---|
| 129 | 10.04 | Patient Materials (filed in section 4.02) | | | | | | | | |
|---|
| 130 | 10.04 | Training/Meeting Materials | | | | | | | | |
|---|
| 131 | 10.04 | Lists/Logs/Vendor Correspondence | | | | | | | | |
|---|
| 132 | 10.05 | Jumo Health | | | | | | | | |
|---|
| 133 | 10.05 | Site Material | N/A | N/A | N/A | N/A | | | | |
|---|
| 134 | 10.05 | Patient materials (filed in section 04.02) | N/A | N/A | N/A | N/A | | | | |
|---|
| 135 | 10.05 | Training/Meeting Materials | N/A | N/A | N/A | N/A | | | | |
|---|
| 136 | 10.05 | Lists/Logs/Vendor Correspondence | N/A | N/A | N/A | N/A | | | | |
|---|
| 137 | 10.06 | Medable/PPD Digital | | | | | | | | |
|---|
| 138 | 10.06 | Medable Site Guide | V1 | 22-Sep-21 | Yes | Yes | | | | |
|---|
| 139 | 10.06 | Training/Meeting Materials | | | | | | | | |
|---|
| 140 | 10.06 | Lists/Logs/Vendor Correspondence | | | | | | | | |
|---|
| 141 | 10.07 | Medidata Rave | | | | | | | | |
|---|
| 142 | 10.07 | eCRF guideline | V1 | 5-Oct-21 | Yes | Yes | | | | |
|---|
| 143 | 10.07 | eCRF guideline | V2 | 2-Dec-21 | | | | | | |
|---|
| 144 | 10.07 | eCRF guideline | V3 | 23-Feb-22 | Yes | Yes | | | | 2-Mar-22 |
|---|
| 145 | 10.07 | Training/Meeting Materials | | | | | | | | |
|---|
| 146 | 10.07 | Lists/Logs/Vendor Correspondence | | | | | | | | |
|---|
| 147 | 10.08 | PRA Safety Reporting System: psiXchange | | | | | | | | |
|---|
| 148 | 10.08 | Reference Guideline V2.0 | V2 | 6-Aug-20 | Yes | Yes | | | | |
|---|
| 149 | 10.08 | Reference Guideline V2.0 | V3 | 4-Nov-20 | | | | | | |
|---|
| 150 | 10.08 | Training/Meeting Materials | | | | | | | | |
|---|
| 151 | 10.08 | Lists/Logs/Vendor Correspondence | | | | | | | | |
|---|
| 152 | 10.09 | Local Department(s)/Third Party Provider(s) | | | | | | | | |
|---|
| 153 | 10.09 | Training/Meeting Materials | | | | | | | | |
|---|
| 154 | 10.09 | Lists/Logs/Vendor Correspondence | | | | | | | | |
|---|
|
|---|